Formula H2O2 Taking the Lead in Disinfection against C. diff. PDI
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2.. H 2 O 2 Lewis Structure. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.
Lewis Dot Diagram For H2o Free Diagram For Student
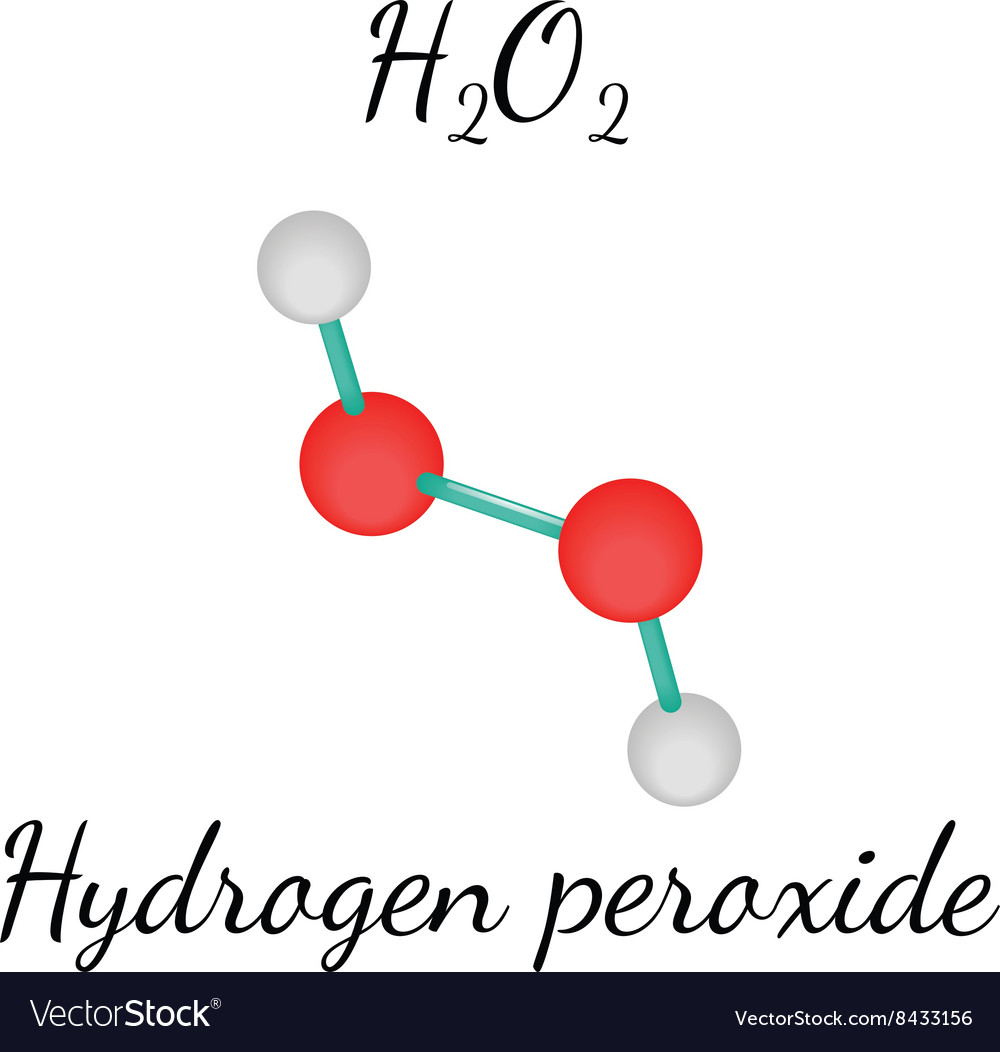
O. 2. ) Lewis Structure. Lewis structure of Hydrogen peroxide (H 2 O 2) contains two O-H bonds and one O-O bond. Also, there are two lone pairs on each oxygen atom. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2. Each step of drawing lewis structure of H 2 O 2 is explained.
Draw lewis struture of H2O2(hydrogen peroxide)
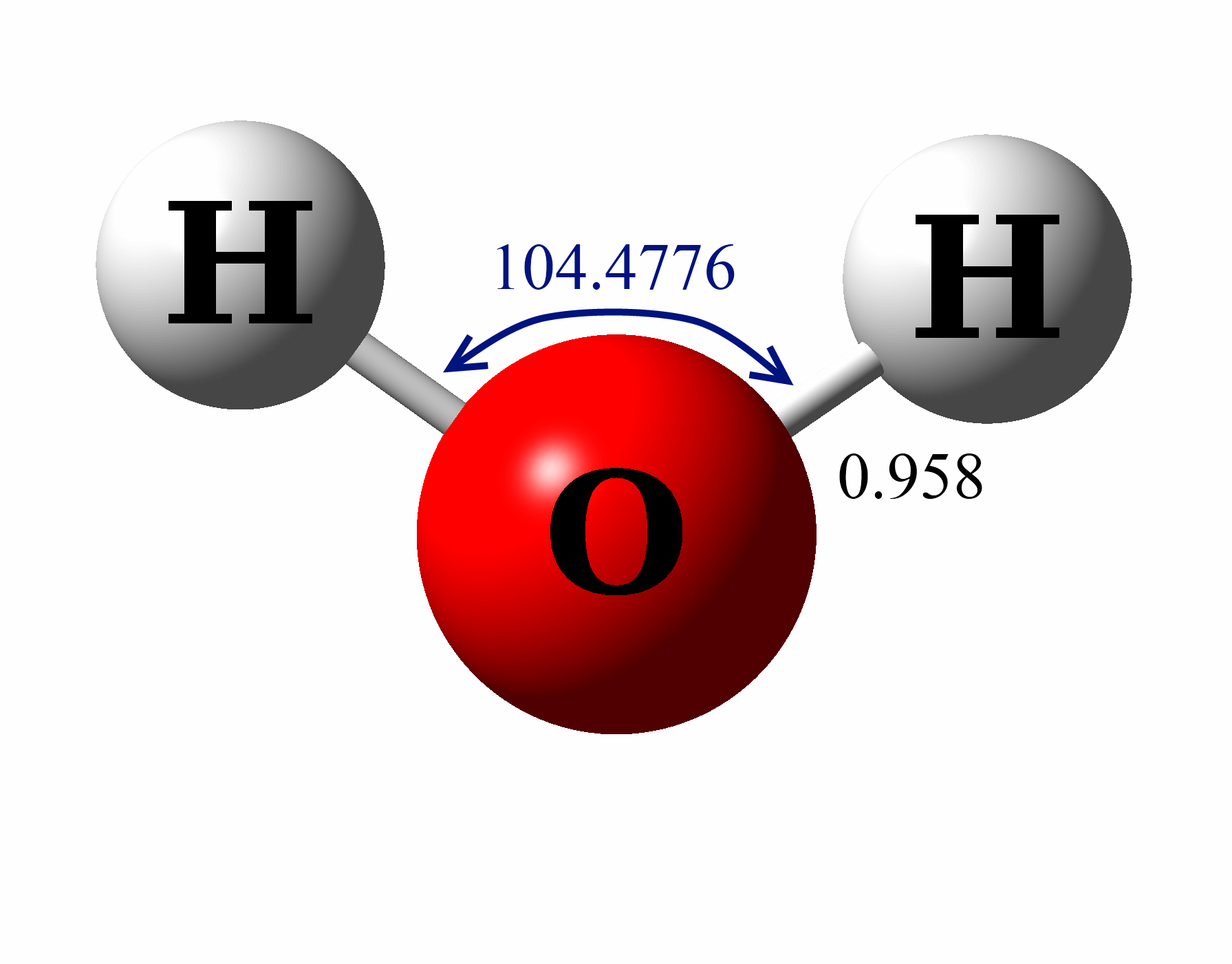
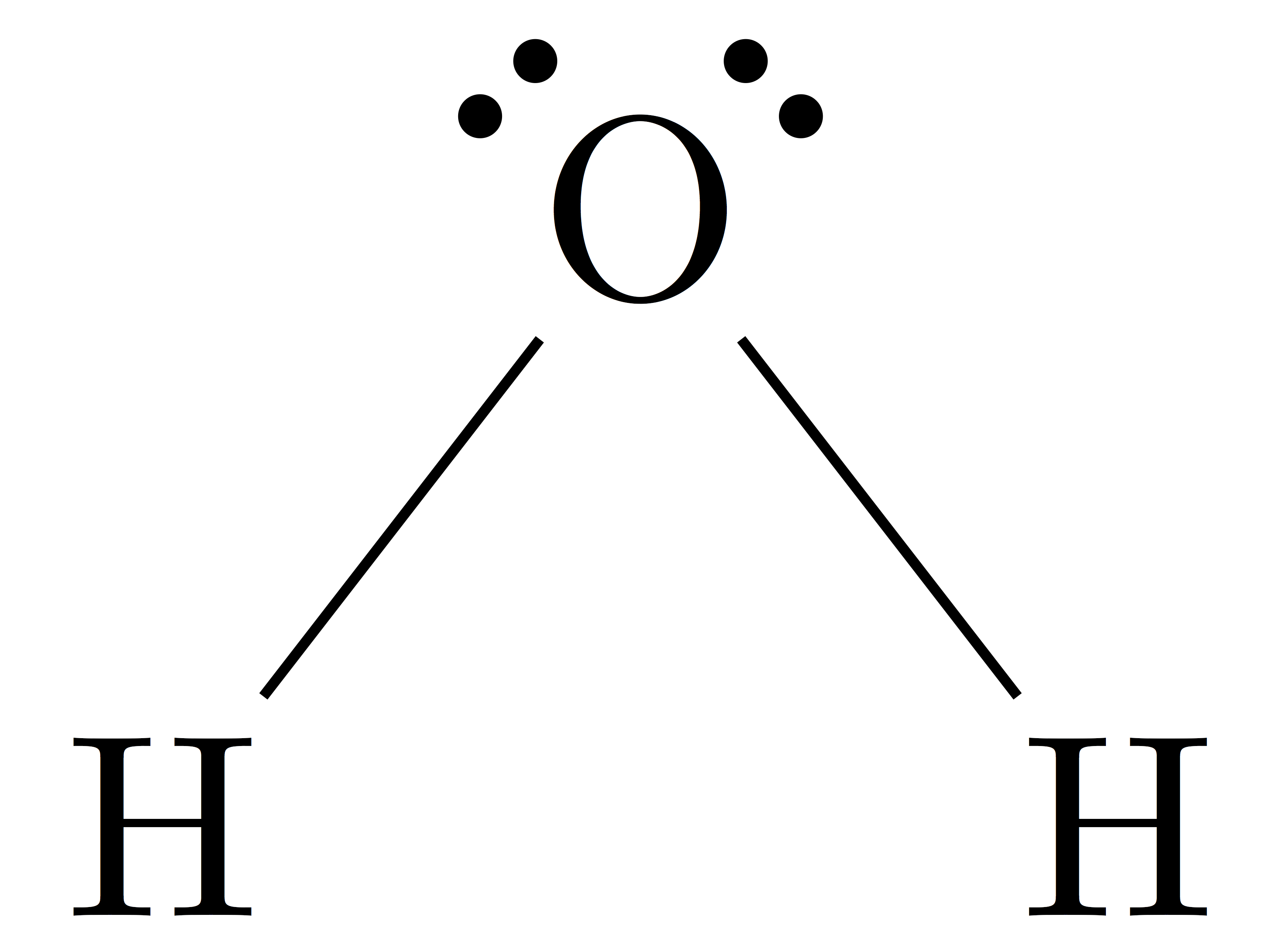
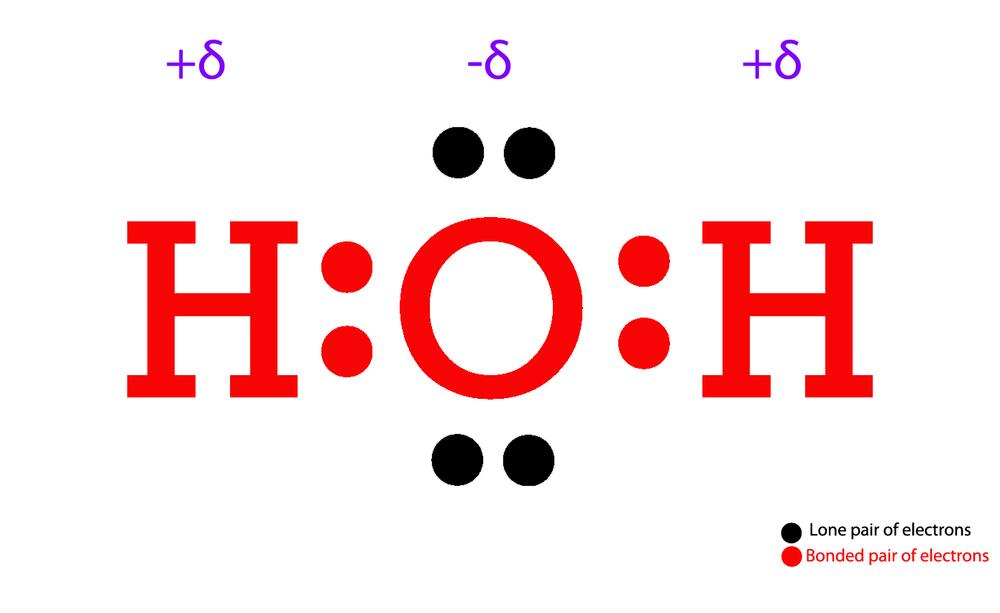
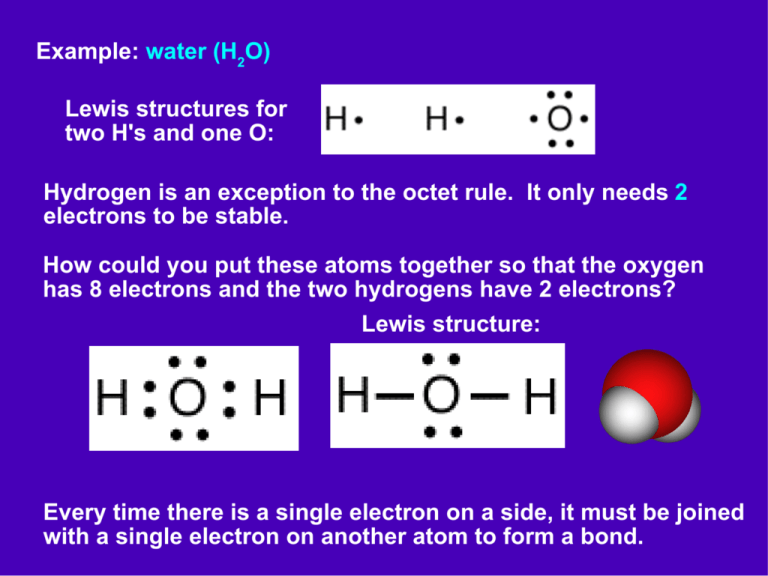
The Lewis Structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule. Because of the two lone pairs, H 2 O will have a bent molecular geometry and it will be a polar molecule. Remember that Hydrogen only needs two electrons to have a full outer shell. Video: Drawing the Lewis Structure.

H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
H2O2 lewis structure is made up of two oxygen and two hydrogen atoms, these atoms made two O-H bonds and one O-O bond. There is a total of 4 lone pairs and 3 bonded pairs are present in the lewis structure of H2O2. The H2O2 lewis dot structure is very simple and the procedure for drawing it same as the other molecules.
2 H2o2 Hot Sex Picture
A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide). Note that the H2O2 Lewis structure is frequently used on tests a.

Lewis Structure of H2O2, Hydrogen Peroxide YouTube
Steps of drawing H2O2 lewis structure Step 1: Find the total valence electrons in H2O2 molecule. In order to find the total valence electrons in H2O2 molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily.

H2O2 Lewis Structure (Hydrogen Peroxide) YouTube
The lewis structure of h2o2 shows that each oxygen atom is bonded to the central hydrogen atom, and each oxygen atom also has an unshared pair of electrons. This arrangement gives hydrogen peroxide its bent molecular shape. In this article, we will explore the lewis structure of h2o2 and its significance in understanding the molecule's.
Hydrogen peroxide solution 95299 Honeywell Research Chemicals
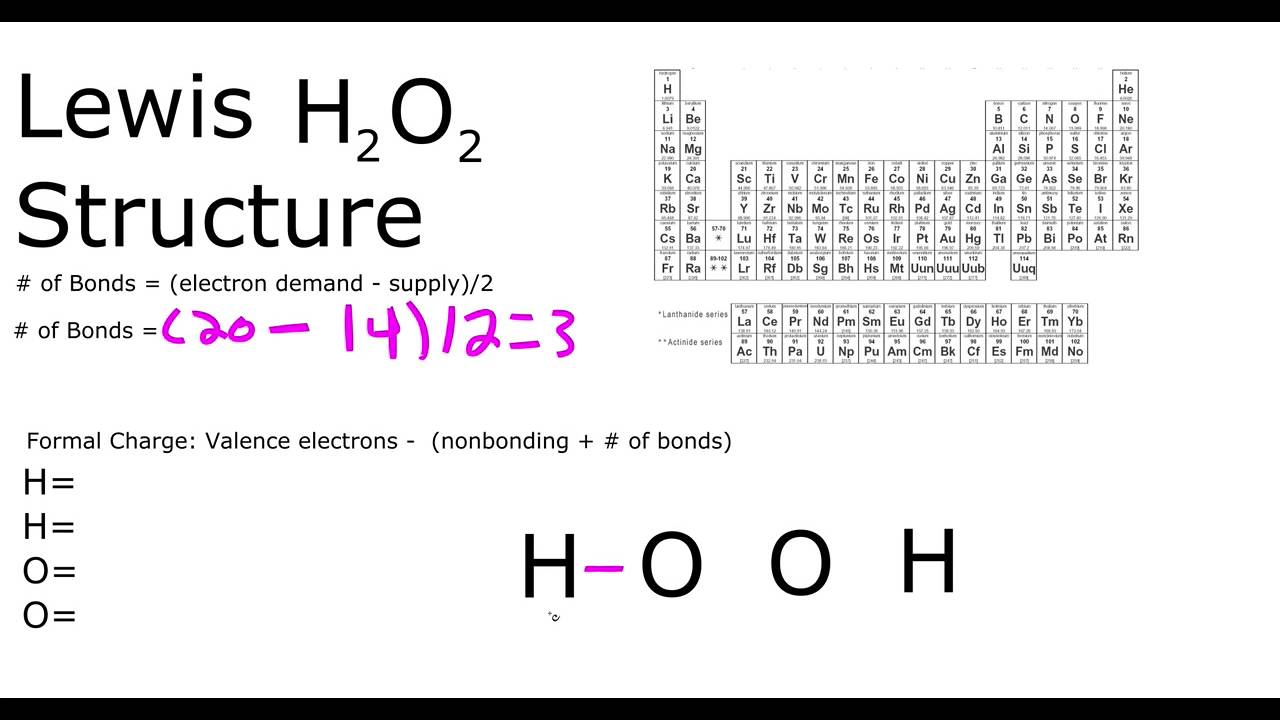
Let's do the Lewis structure for H2O2: Hydrogen Peroxide, also called dihydrogen dioxide. On the periodic table, Hydrogen's in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2. Oxygen, group 6 or 16, we have two of those, so let's multiply that by 2 as well for a total of 14 valence electrons. Let's.
H2O2 Lewis Structure YouTube
A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide).For the H2O2 structure use the periodic table to find the total nu.
H2o Lewis Structure Shape
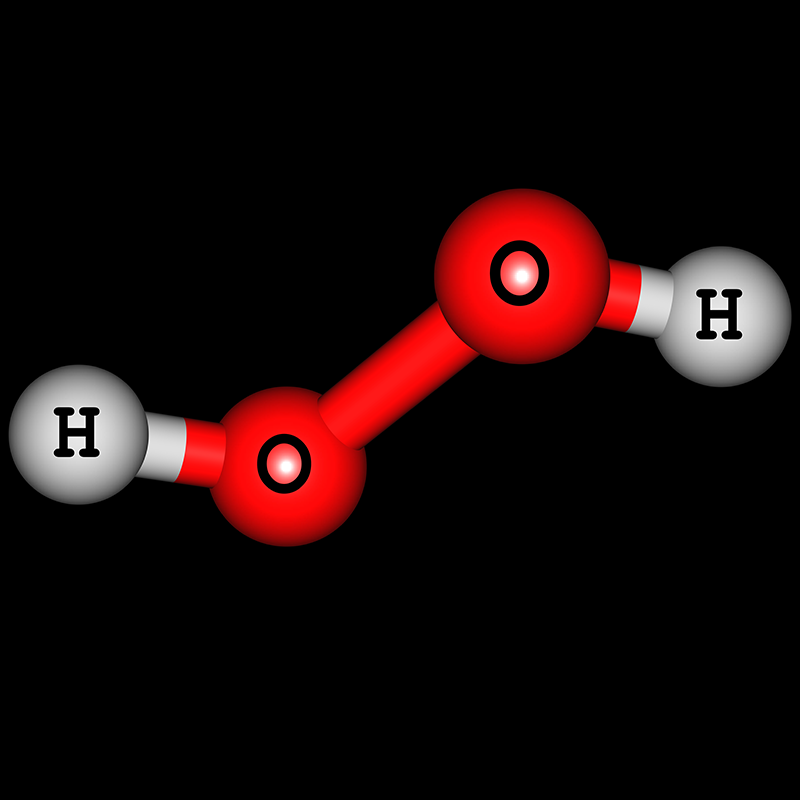
H2O2, hydrogen peroxide is a funny looking molecule: It has two oxygen atoms in the centre, and they end up single-bonded. Then, there is a Hydrogen Atom at.

Is H2O2 Polar or Nonpolar? Techiescientist
H 2 O 2 Lewis structure. H 2 O 2 (hydrogen peroxide) has two hydrogen atoms and two oxygen atoms. In the H 2 O 2 Lewis structure, there is a single bond between the two oxygen atoms, and each oxygen is attached with one hydrogen atom, and on each oxygen atom, there are two lone pairs. Steps. #1 Draw a rough skeleton structure.

Structure of h2o2? explain with diagram Brainly.in
To draw the Lewis structure of H2O2, we need to follow a few simple steps: Step 1: Count the total number of valence electrons in the molecule. H2O2 has 2 hydrogen atoms, 2 oxygen atoms, and 4 valence electrons (6 for oxygen and 1 for hydrogen). Therefore, the total number of valence electrons in H2O2 is 2 × 1 + 2 × 6 = 14.
Draw Step By Step The Lewis Structure For Water (H2O)
Hydrogen peroxide is a chemical compound with the formula H 2 O 2.In its pure form, it is a very pale blue liquid that is slightly more viscous than water.It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%-6% by weight) in water for consumer use, and in higher concentrations for industrial use.Concentrated hydrogen peroxide, or "high-test peroxide.

Lewis Structure Hydrogen Peroxide H2o2 Scientific Stock Vector (Royalty
H2O2 Lewis Structure. We shall discuss the chemical bonding nature of H2O2 in this article. First, we shall draw the Lewis structure of H2O2. A Lewis structure may not give us a complete description of the molecular geometry. Still, it is helpful to visualize the overall skeletal structure of the molecule, its bonds, and its lone pairs.

Hydrogen Peroxide Chemical Structure H2o2 Stock Vector (Royalty Free
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.
Lewis structure
Hello Guys!Hydrogen Peroxide or Dihydrogen Dioxide consists of two hydrogen atoms and two oxygen atoms. This video on H2O2 Lewis Structure will help you unde.